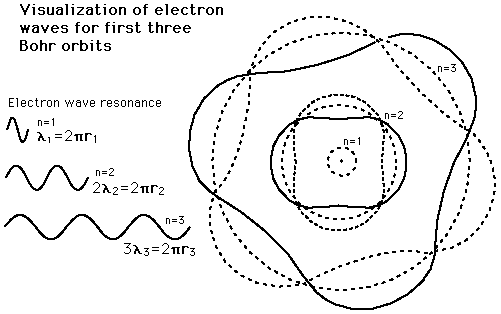
IIRC, this theory is quite old and there were a lot of new advances since it was introduced(quantum mechanics and whatnot).
Is the system that simple in reality, or is it an easy to explain dumbed-down version? If so, what's really happening "down there"?
Is the system that simple in reality, or is it an easy to explain dumbed-down version? If so, what's really happening "down there"?








Comment